Submitted by Carmyn de Jonge on Tue, 01/09/2020 - 10:19
Can Healthy Ecosystems Prevent Future Pandemics?
Written by Charlotte Milbank, Interdisciplinary PhD Candidate. Geography and Epidemiology.
The COVID-19 pandemic has thrown deserved spotlight on the complex interactions and interdependencies between environmental health and human health in relation to the risk of zoonotic disease emergence. This piece was motivated by a virtual panel convened by the Conservation Research Institute, Cambridge Institute for Public Heath, the Infectious Disease IRC and the Global Food Security IRC, held on 14th July 2020, which asked ‘can healthy ecosystems prevent future pandemics’? Here, we discuss key insights from this panel, arguing that truly interdisciplinary approaches are needed if we are to effectively mitigate zoonotic risk and protect both ecological and human health in the future. The panel included:
- Dr Shailaja Fennell (Development Studies, Department of Land Economy)
- Professor Kate Jones (Chair of Ecology & Biodiversity, Division of Biosciences, University College London)
- Dr Rosalind Parkes-Ratanshi (Cambridge Institute for Public Heath)
- Professor James Wood (Department of Veterinary Medicine, Cambridge Infectious Diseases)
- Professor Bhaskar Vira, (Panel Chair; Geography and founder of the Conservation Research Institute).
The SARS-CoV-2 pandemic has forced change upon the way we live as humans in 2020. To date, over 20 million people have been infected with COVID-19 across the world, with no sign of any immediate abatement. Understanding the zoonotic causes of COVID-19’s emergence and the conditions that might lead to similar future pandemics is of urgent concern. Increasing evidence suggests that conditions within degraded ecosystems are ripe for zoonotic spillover and spread. Drawing on expertise from diverse disciplines housed in the CCI, this panel was well-placed to discuss this topic and broach what the implications of this might be for future research and effective policy response.
Zoonotic Spillover
Zoonotic diseases (or "zoonoses") are diseases that normally exist in (non-human) animals but can “jump” to infect humans in so-called ‘spillover’ events. Transmission from animal to human may be airborne, through food, close or direct contact with animals, their faeces or saliva, or vector-borne. 60% of existing infectious diseases are of zoonotic origin or have transmission pathways involving animals, and 75% of future emerging diseases are expected to come from animals. Zoonotic spillover is common and the majority of spillover events go unreported. Many neglected zoonotic diseases (such as bovine tuberculosis, brucellosis and plague) remain in regular circulation in some human populations, although it is rare that such diseases go on to cause pandemics
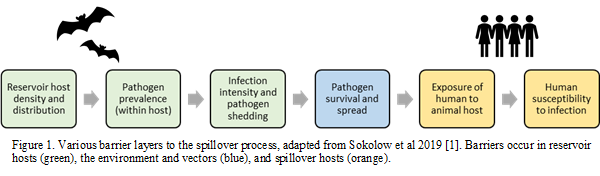
Spillover from animals to humans requires the infectious pathogen to overcome a succession of several disease-related, animal, human and environmental barriers, namely the simultaneous co-existence of an animal host species, sufficient prevalence of pathogen and infection intensity within that animal host, animal exposure to a susceptible human population, and pathogen survival within humans. With new anthropogenic pressures and behaviours, animals and humans are being brought into closer contact than ever before, and bringing with it new risks of zoonotic emergence. If we can understand the pathways to spillover events, we may be better placed to intervene at several points along these pathways, and mitigate the risk of spillover occurrence.
Spillover in Degraded Ecosystems
Whilst the animal hosts of zoonotic disease are often heavily focused on, they are just one part of the spillover story. Human behaviour is inextricably linked to spillover events. A recent review of all 183 documented zoonotic pathogens since 1940 found that 31% of reported zoonotic spillover events could be linked to anthropogenic land use change, such as deforestation, land clearance for human settlements, industrial agriculture, mining and other infrastructure [2]. The degradation of natural environments associated with these changes influences the distribution and breeding patterns of both pathogens and animal hosts, and often brings humans and animals into closer contact. This creates new and increased opportunities for spillover [3,4]. Citeable examples include findings that increased deforestation and land use modification in the Amazon significantly increased the biting rate of malaria-carrying mosquitoes, Anopheles darlingi [5,6]. Landscape fragmentation has also been associated with pathogen prevalence and zoonotic spillover of tick-borne encephalitis in Latvia [7] and West Nile Virus in Southern France [8]. As we continue to see record losses of ecosystems and biodiversity, understanding how these changes may influence patterns of contact between pathogens, animal hosts and humans will be important to limiting the risks of spillover.
Conditions for Transmission
Spillover events are comparatively commonplace compared to the actual take-off and widespread transmission of zoonotic disease within human populations. Take-off depends on whole other suite of pathogenic, human, environmental factors that create opportunities for transmission. For example, does the pathogen have high environmental stability? Is it highly infectious? And how does it spread? Are humans coming into sufficient, regular contact to allow transmission? And are there important environmental factors, such as those associated with environmental degradation, that mediate these relationships?
One panellist used the initial spread of human immunodeficiency virus (HIV) through Africa in the late 1900s as a useful example, illustrating again the need to look beyond animal and disease factors, and towards human behaviour. A zoonotic disease of primate origin, HIV is thought to have spilt-over to humans in western Africa, spreading initially through human populations via train, truck and trade routes across the continent. Such travel routes bring otherwise-distant populations ‘into contact’ with each other. In an ever-globalised world, the spread of COVID-19 - a highly transmissible virus with moderate environmental stability - has also been facilitated by widespread human movement. Understanding human behaviours and the conditions that are ripe for emerging zoonotic diseases to take off with onward human transmission is as important as understanding the biology of spillover when it comes to designing effective strategies of prevention.
Separating Humans and Nature?
Given we know that the degradation of ecosystems is associated with zoonotic disease emergence and transmission, what can we do to reduce these risks? The obvious solution might seem to target the separation of humans and nature. When broached with this question, panellists suggested that total separation was not the answer going forward, first and foremost because it is an impossibility - there is no intact ecosystem in the world that does not have some degree of human interference. Furthermore, exclusionary policies often have unintended negative consequences that often disproportionately affect already-marginalised populations. In India, the Ministry for Environment and Forests has instructed all states to seek to reduce human-wildlife interaction by placing restrictions on access to National Parks, sanctuaries and Tiger Reserves. This directive applies to 3-4 million (mostly tribal) people who live proximate to these areas, and who often rely on these areas for natural subsistence resources. Elsewhere in Asia, legislation similarly trying to reduce opportunities for human-wildlife contact, including bans on wildlife trade and consumption, have been enacted elsewhere in Asia.
If separation is not the answer, we need to develop better understanding of the ways that humans interact with nature in spaces at risk of spillover. And given that spillover is more likely in degraded spaces, efforts should be made to restore these landscapes.
Compounding/Stacking of Disasters
Attempts at solutions must also pay heed to the heterogenous needs and vulnerabilities of communities that may be adversely affected by COVID-19 and future emerging zoonoses. Whilst COVID-19 has certainly been a shock for us all, one panellist drew important attention to parts of the world experiencing a 'stacking of shocks', citing the swarms of locusts currently plaguing dozens of countries, including Kenya, Ethiopia, Uganda and India as an example. In communities here, the threat of COVID-19 compounds the ongoing threat that communities face to their daily food security, as cropland, grazing land and home environments are ravaged by the insects. The pandemic has also increased the vulnerability of displaced populations, who may already inhabit areas that are unsafe, lack basic health infrastructure and coping mechanisms, and are now subject to COVID-19 restrictions. There is need to affirm our understandings of the diverse impacts of COVID-19 on communities worldwide, and recognise that a one-size-fits-all policy response is rarely appropriate.
Adopting a ‘One Health’ Approach
All this points to the need for decisive, interdisciplinary action. The ‘One Health’ approach defined by the WHO [9] recognises the need for collaboration of expertise spanning multiple disciplines to drive effective research and policy responses. Such collaboration would enable recognition and understanding of the complex entanglement of pathogenic, human, animal and the environmental factors that influence the emergence, spread and heterogeneous impacts of zoonotic disease.
That the degradation of environments increases the risk of zoonotic spillover and transmission, and pose threat to human health, is now widely accepted. But we cannot hope to mitigate these risks and threats by continuing to look at any one component (human, animal, pathogen, environment) in isolation. The emergence and ongoing threat of COVID-19 calls for a united response from ecologists, veterinary scientists, epidemiologists, public health practitioners, social scientists – as well as a response that is grounded in understanding of the needs and vulnerabilities of diverse local communities.
Please click here to view the Event recording
References:
1. Sokolow et al., (2019) ‘Ecological interventions to prevent and manage zoonotic pathogen spillover’, Philosophical Transactions of the Royal Society B: Biological Sciences. Royal Society Publishing. doi: 10.1098/rstb.2018.0342.
2. Loh, E. H. et al. (2015) ‘Targeting Transmission Pathways for Emerging Zoonotic Disease Surveillance and Control’, Vector-Borne and Zoonotic Diseases. Mary Ann Liebert Inc., 15(7), pp. 432–437. doi: 10.1089/vbz.2013.1563.
3. Azevedo, J. C. et al. (2020) ‘The ethics of isolation, the spread of pandemics, and landscape ecology’, Landscape Ecology. Springer, pp. 1–8. doi: 10.1007/s10980-020-01092-8.
4. Lambin, E. F. et al. (2010) ‘Pathogenic landscapes: Interactions between land, people, disease vectors, and their animal hosts’, International Journal of Health Geographics. BioMed Central, 9, p. 54. doi: 10.1186/1476-072X-9-54.
5. MacDonald, A. J. and Mordecai, E. A., (2019) Amazon deforestation drives malaria transmission, and malaria burden reduces forest clearing. Proc Natl Acad Sci USA 116(44):22212–22218
6. Vittor AY, Gilman RH, Tielsch J, Glass G, Shields T, Lozano WS, Pinedo-Cancino V, Patz JA (2006) The effect of deforestation on the human-biting rate of Anopheles dar- lingi, the primary vector of Falciparum malaria in the Peruvian
7. Vanwambeke, S. O. et al. (2010) ‘Landscape predictors of tick-borne encephalitis in Latvia: Land cover, land use, and land ownership’, Vector-Borne and Zoonotic Diseases. Mary Ann Liebert Inc., 10(5), pp. 497–506. doi: 10.1089/vbz.2009.0116.
8. Pradier, S., Leblond, A. and Durand, B. (2008) ‘Land cover, landscape structure, and West Nile virus circulation in southern France’, Vector-Borne and Zoonotic Diseases. Mary Ann Liebert Inc., 8(2), pp. 253–263. doi: 10.1089/vbz.2007.0178.
9. World Health Organisation (2017) One Health. [online] Available at: <https://www.who.int/westernpacific/news/q-a-detail/one-health> [Accessed 19 August 2020].